You may have heard of carbon tetrachloride before, but did you know that this compound has a unique relationship with water?
Yes, you read that right. The two substances can actually interact and potentially dissolve into each other.
Sounds like some kind of magic, right? Well, it’s not quite as mystical as it sounds.
In fact, it’s all thanks to some fascinating chemistry principles at play. So if you’re ready to take a deep dive into the world of solubility and learn about the potential of carbon tetrachloride in water, then let’s get started.
Trust us, this is one blog post you won’t want to miss.
Can Carbon Tetrachloride Dissolve In Water?
Contents
- 1 Can Carbon Tetrachloride Dissolve In Water?
- 2 The Ban on Carbon Tetrachloride and its Effects on Human Health and the Environment
- 3 Can Carbon Tetrachloride Dissolve in Water?
- 4 Factors Affecting Solubility of Carbon Tetrachloride in Water
- 5 Proper Disposal of Solutions Containing Carbon Tetrachloride
- 5.1 How to Properly Dispose of Solutions Containing Carbon Tetrachloride
- 5.2 Consult with local authorities or waste management companies
- 5.3 Seek out a licensed hazardous waste disposal facility
- 5.4 Chemically neutralize the solution before disposal
- 5.5 Never pour solutions down the drain or into the soil
- 6 Dangers of Improper Disposal and Contamination of Water Sources
- 7 How to Handle Carbon Tetrachloride Safely to Prevent Harmful Effects
- 8 Conclusion
When we think about disposing of harmful chemicals, we often consider their potential impact on the environment. One such chemical that has been a cause for concern is carbon tetrachloride. This colorless liquid has been used in various industrial and commercial applications, but its potential to dissolve in water has raised red flags among environmentalists and health experts. So, let’s explore the solubility of carbon tetrachloride in water and its potential impact on the environment.
Firstly, what exactly is solubility? In simple terms, it is the ability of a substance to dissolve in another substance, known as a solvent. In the case of carbon tetrachloride, water is the solvent. And the short answer to whether or not carbon tetrachloride can dissolve in water is yes.
But there’s more to it than just a simple yes or no answer. The solubility of carbon tetrachloride in water depends on various factors such as temperature, pressure, and concentration. At room temperature, only a small amount of carbon tetrachloride can dissolve in water, with most of it remaining separate from the water and forming a layer on top.
However, if the temperature is increased or pressure is applied to the mixture, the solubility of carbon tetrachloride also increases. At boiling point, this chemical can become fully miscible with water, meaning they can mix evenly and form a single solution. This is why it is crucial to handle and dispose of this chemical carefully, as it can easily dissolve in hot water and contaminate it.
Moreover, if there are already other substances present in the water or if the carbon tetrachloride solution is highly concentrated, its solubility in water can also increase. This poses a significant risk to aquatic life and human health if large amounts of this chemical are released into water sources.
The Ban on Carbon Tetrachloride and its Effects on Human Health and the Environment
“Say Goodbye to Carbon Tetrachloride: How to Properly Dispose of Products Containing This Harmful Chemical”
As the saying goes, out of sight, out of mind. But when it comes to the harmful chemical carbon tetrachloride, just because we can’t see it doesn’t mean it’s not there. This colorless liquid, once widely used in various industries, has been banned since the 1970s due to its damaging effects on human health and the environment. But did you know that improper disposal of products containing this chemical can still pose a threat? Here’s what you need to know about carbon tetrachloride and how to properly dispose of it.
Firstly, let’s talk about the potential dangers of exposure to carbon tetrachloride. This chemical can enter our bodies through inhalation, skin contact, or ingestion. The main route of exposure for humans is through inhalation, which can lead to a range of health issues such as liver and kidney damage, neurological effects, and even cancer. And it’s not just humans at risk – when released into the environment, carbon tetrachloride can contaminate soil and water sources, posing a threat to aquatic life as well.
But enough doom and gloom – let’s focus on what we can do to prevent these risks. The most important step is proper disposal of any products containing carbon tetrachloride. This includes old fire extinguishers, cleaning agents, and even some pesticides. Here are some tips to ensure safe disposal:
- Check the label: Before disposing of any product, check the label for any warnings or instructions on how to handle and dispose of it properly. If it contains carbon tetrachloride, take extra precautions.
- Contact your local hazardous waste facility: Most communities have designated facilities for the disposal of hazardous waste. Contact them to find out the proper procedures for disposing of products containing carbon tetrachloride in your area.
- Do not pour down the drain: It may be tempting to simply pour old cleaning agents down the drain, but this can lead to contamination of water sources. Instead, take them to a hazardous waste facility or contact your local waste management company for guidance.
- Seal and label: When disposing of any product containing carbon tetrachloride, make sure it is securely sealed and clearly labeled as hazardous waste. This will prevent accidental exposure and ensure proper handling.
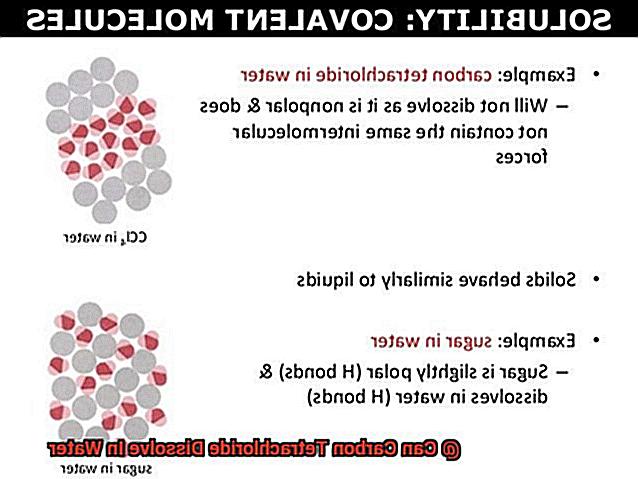
Can Carbon Tetrachloride Dissolve in Water?
If you’ve ever used a spot remover or a fire extinguisher, chances are you’ve come across carbon tetrachloride. This colorless liquid is commonly used in industrial processes and can also be found in some consumer products. However, its potential for environmental pollution has raised concerns and sparked the question: can carbon tetrachloride dissolve in water?
The answer is yes, but it’s not a straightforward process. To understand how carbon tetrachloride dissolves in water, we first need to understand its chemical properties. Carbon tetrachloride is a nonpolar compound, meaning it does not have a positive or negative charge. On the other hand, water is a polar compound, with a positive charge on one end and a negative charge on the other. This means that water molecules are attracted to each other and can also interact with other polar compounds.
When carbon tetrachloride is added to water, it does not readily dissolve. This is because the nonpolar molecules of carbon tetrachloride are not attracted to the polar molecules of water. Instead, they clump together and form droplets on top of the water. So, if you were to pour carbon tetrachloride into a glass of water, you would see it floating on top rather than mixing in.
However, with enough agitation or stirring, some of the carbon tetrachloride molecules may be able to break free from the clumps and mix with the water. This process is known as dispersion. It’s important to note that only a small amount of carbon tetrachloride can dissolve in water this way.
Temperature also plays a role in the dissolution of carbon tetrachloride in water. As the temperature of the water increases, so does the solubility of carbon tetrachloride. This means that more carbon tetrachloride molecules can dissolve in warm water compared to cold water.
The pH of the water can also affect the dissolution process. In acidic conditions, carbon tetrachloride is more soluble in water compared to neutral or alkaline conditions. This is because acidic solutions have a lower amount of hydrogen bonds, making it easier for nonpolar molecules like carbon tetrachloride to mix in.
Factors Affecting Solubility of Carbon Tetrachloride in Water
You may have heard the phrase “like oil and water” used to describe two things that just don’t mix. But did you know that there is a chemical that actually behaves like this in real life? Carbon tetrachloride (CCl4) is a colorless liquid that was once commonly used as a solvent, but has now been banned due to its harmful effects on human health and the environment.
One of the most interesting aspects of carbon tetrachloride is its solubility, or ability to dissolve, in water. On one hand, carbon tetrachloride is a nonpolar compound, meaning it lacks an electric charge and does not mix well with polar substances like water. However, in theory, it should be able to dissolve in water due to its small molecular size and ability to form hydrogen bonds with water molecules.
So, what factors affect the solubility of carbon tetrachloride in water? Let’s take a closer look.
- Temperature: As with most substances, the solubility of carbon tetrachloride in water increases with higher temperatures. This is because higher temperatures provide more energy for the molecules to overcome their intermolecular forces and mix together.
- Pressure: Increasing the pressure can also increase the solubility of carbon tetrachloride in water, as it forces more molecules into a smaller space, making them more likely to interact and dissolve.
- Presence of other solutes: If there are already other substances dissolved in the water, there may be less room for carbon tetrachloride molecules to fit and dissolve. Additionally, the polarity of other substances in the solution can also play a role. Polar substances like salts or sugars can decrease the solubility of nonpolar substances like carbon tetrachloride by competing for interactions with water molecules.
- Purity: Impurities in either the carbon tetrachloride or water can greatly affect their solubility. These impurities can disrupt the molecular interactions and decrease solubility.
- Physical agitation: Finally, physical agitation or stirring can also enhance the solubility of carbon tetrachloride in water. By breaking up the surface tension of the water, more carbon tetrachloride molecules can come into contact with the water molecules and dissolve.
Proper Disposal of Solutions Containing Carbon Tetrachloride
How to Properly Dispose of Solutions Containing Carbon Tetrachloride
If you have ever worked with carbon tetrachloride, you know that it is not something to be taken lightly. This chemical compound was once commonly used as a solvent, degreaser, and fire extinguisher, but due to its harmful effects on both human health and the environment, its use has been greatly restricted. One of the main concerns with carbon tetrachloride is its ability to dissolve in water. This means that if it is not properly disposed of, it can easily contaminate water sources and potentially harm aquatic life.
So, what can we do to prevent this from happening? Let’s take a closer look at some of the best practices for disposing of solutions containing carbon tetrachloride.
Before disposing of any solution containing carbon tetrachloride, it is crucial to consult with local authorities or waste management companies for guidance. They will be able to provide specific instructions on how to safely dispose of the solution and may even offer hazardous waste disposal services.
Handle and store solutions containing carbon tetrachloride carefully
It is important to handle and store solutions containing carbon tetrachloride with caution. The compound is highly flammable and can also release toxic fumes if not stored properly. Keep it in a well-ventilated area away from sources of heat or ignition.
Seek out a licensed hazardous waste disposal facility
One option for disposing of solutions containing carbon tetrachloride is to seek out a licensed hazardous waste disposal facility. These facilities are equipped to handle and dispose of hazardous materials safely.
Chemically neutralize the solution before disposal
Another option is to chemically neutralize the solution before disposing of it. This can be done by adding a reducing agent such as sodium bisulfite or sodium sulfite to the solution. However, this should only be attempted by trained professionals as it can be dangerous if not done correctly.
Never pour solutions down the drain or into the soil
It is important to note that pouring solutions containing carbon tetrachloride down the drain or into the soil is never acceptable. This can lead to contamination of water sources and harm to both humans and wildlife.
Dangers of Improper Disposal and Contamination of Water Sources
Have you ever heard of carbon tetrachloride? This highly toxic and dangerous chemical may not be on your radar, but it should be. Improper disposal and contamination of water sources with this chemical can have severe consequences for both human health and the environment.
So what exactly is carbon tetrachloride? It is a colorless, volatile liquid that was commonly used in industries such as dry cleaning and fire extinguisher production. However, due to its toxic nature, it has been banned in many countries. But the lingering effects of improper disposal in the past are still being felt today.
One of the most concerning dangers of carbon tetrachloride is its impact on human health. Exposure to this chemical has been linked to liver and kidney damage, as well as reproductive issues. It is also classified as a known carcinogen, meaning it can cause cancer. This is why proper disposal methods are crucial in preventing its harmful effects on our health.
But the dangers don’t stop there. When carbon tetrachloride is released into water sources, it can quickly spread and contaminate large areas. This poses a significant threat to aquatic life, leading to the death of plants and animals. And if that contaminated water is consumed or used for daily activities like bathing or washing, it can have long-term effects on human health.
The economic impact of carbon tetrachloride contamination cannot be ignored either. Contaminated water cannot be used for irrigation or industrial purposes, resulting in financial losses for farmers and businesses. This not only affects their livelihoods but also impacts industries that rely on clean water sources.
Furthermore, improper disposal of carbon tetrachloride can also lead to the pollution of groundwater – a vital source of drinking water for many communities. This could result in costly cleanup efforts and potential legal consequences for those responsible for the pollution.
So what can we do to prevent the dangers of carbon tetrachloride? Proper disposal is key. This means consulting with authorities and utilizing licensed hazardous waste disposal facilities. It may also require stricter regulations and enforcement to ensure that industries are not cutting corners when it comes to disposing of this chemical.
How to Handle Carbon Tetrachloride Safely to Prevent Harmful Effects
Carbon tetrachloride, also known as CCl4, is a chemical compound that was commonly used in the past as a solvent for cleaning and degreasing purposes. However, due to its toxic nature, its use has been restricted by the Environmental Protection Agency (EPA). In this blog post, we will discuss the risks associated with handling carbon tetrachloride and provide tips on how to safely dispose of it as hazardous waste.
The Risks of Carbon Tetrachloride:
One of the main concerns with carbon tetrachloride is its ability to dissolve in water. This makes it highly dangerous when it comes to disposal methods, as it can contaminate water sources and pose a risk to both humans and aquatic life. Exposure to carbon tetrachloride can result in serious health issues such as liver and kidney damage, respiratory problems, and even cancer.
Proper Handling and Disposal Methods:
To ensure safety and prevent any negative impact on our health and the environment, it is crucial to handle carbon tetrachloride safely. This includes avoiding any contact with skin or inhalation of fumes, wearing protective gear such as gloves, goggles, and a mask, and following EPA guidelines for its disposal. Carbon tetrachloride should never be poured down the drain or thrown in the regular trash. It should be treated as hazardous waste and disposed of according to local regulations.
Alternative Solutions:
The best way to handle carbon tetrachloride is to avoid using it altogether. There are safer alternatives available in the market for the same purposes. If you do have to handle or dispose of carbon tetrachloride, it is essential to educate yourself on the proper handling methods and take necessary precautions.
The Importance of Prevention:
Prevention is always better than cure when it comes to handling toxic substances like carbon tetrachloride. Educating yourself on the dangers of this chemical and proper handling methods can go a long way in protecting yourself and the environment. By taking the necessary precautions and following EPA guidelines, we can prevent contamination of water sources and minimize the risks associated with carbon tetrachloride.
Conclusion
In conclusion, the intriguing relationship between carbon tetrachloride and water is a result of the intricate principles of chemistry. As we have discovered, this compound can indeed dissolve in water, but its solubility is influenced by various factors such as temperature, pressure, and concentration.
However, it is crucial to acknowledge the potential harm that carbon tetrachloride can cause to our health and the environment. Improper disposal of products containing this chemical can lead to contamination of water sources and pose a threat to aquatic life. Therefore, it is essential to handle and dispose of this compound with caution and adhere to proper guidelines to prevent any negative impacts.
The ban on carbon tetrachloride serves as a reminder of the importance of being mindful of the chemicals we use and their potential effects on our surroundings. By taking steps to properly dispose of this harmful substance, we can protect ourselves and the environment from its dangers.
Remember, when dealing with toxic substances like carbon tetrachloride, prevention is always better than cure. Let us educate ourselves on proper handling methods and take necessary precautions for a safer future for all.